
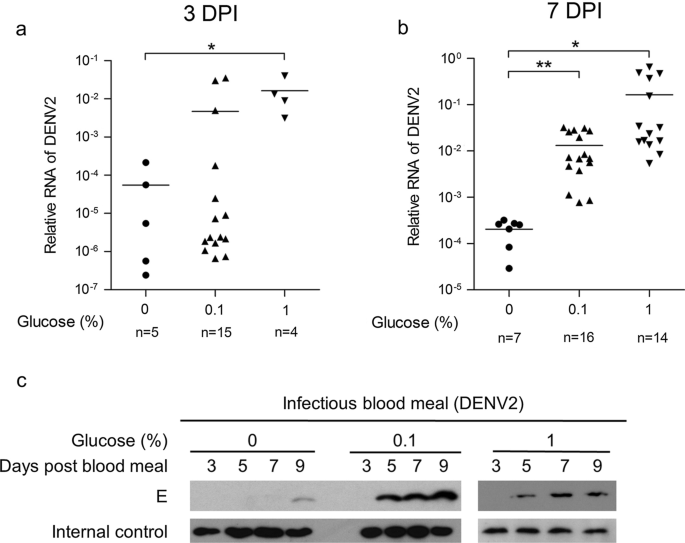
Therefore, IDH mutations may be causally linked to the clinical impacts of patients with MDS. These enzymes are associated with diverse cellular processes such as adapting to histone deacetylation, hypoxia, and DNA demethylation. IDH 1/2 are key metabolic enzymes that convert isocitrate to α-ketoglutarate (α-KG or 2-oxoglutarate, 2-OG), which is an essential cofactor for α-KG dependent dioxygenases. Therefore, contributing gene mutations may supplement current prognostic systems to improve the prediction of prognosis for MDS patients. Genetic mutations are not currently used in estimating prognosis in MDS but are likely key determinants of overall survival and clinical phenotypes. Until now, the pathogenesis of MDS has not been clearly identified, but it is generally acknowledged that genetic mutations and dysfunction of gene contribute to the development and progression of this preleukemic disease. Hence, novel molecular markers may offer more precise cancer phenotypes and more accurate estimation of prognosis for MDS patients. Although existing systems such as the IPSS, Revised-IPSS and WHO-classification-based Prognostic Scoring System (WPSS) help to estimate patient outcomes and guide treatment decisions, there remains significant variability in prognosis. Current prognostic scoring systems for patients with MDS are mainly based on karyotypic abnormalities and certain clinical features that are used to stratify risk. Despite recent advances in therapeutic methods, treatments for MDS are currently tailored to individual patient needs, making the precise forecast of the prognosis an important component of treating patients. Myelodysplastic syndromes (MDS) comprise a heterogeneous group of hematological disorders defined by blood cytopenias due to ineffective hematopoiesis and an increased risk of developing acute myeloid leukemia (AML).


 0 kommentar(er)
0 kommentar(er)
